February 1, 2023. – Toronto, ON: LifeLabs is pleased to share the launch of SignateraTM, a highly sensitive, personalized molecular residual disease assay (MRD) test developed by Natera for treatment monitoring and molecular residual disease (MRD) assessment in patients previously diagnosed with cancer.
The first of its kind, this innovative test uses circulating tumor DNA (ctDNA) and is personalized for each patient to help assess recurrence risk and identify relapse up to two years earlier than the current standard of care tools. The clinical utility of SignateraTM across cancer types has been validated by multiple studies. In those trials, SignateraTM demonstrated predictive values such as1-4:
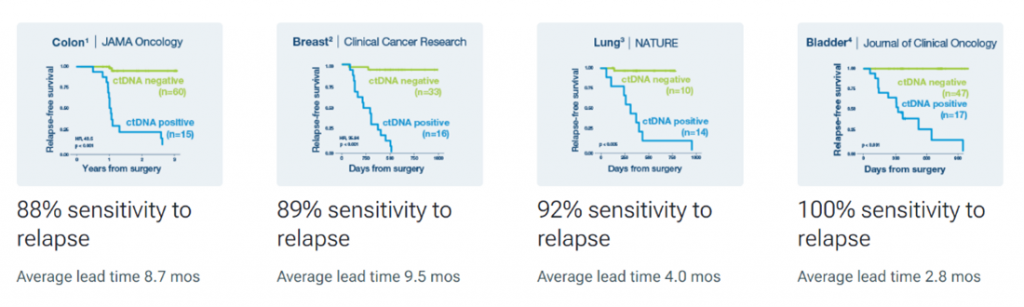
“Diagnosis of diseases such as cancer are complex and unique to each patient,” said Lawrence Mahan, SVP of Commercial, Business and Consumer Markets for LifeLabs. “It’s important to us at LifeLabs that we continue to grow in a way that expands access to important health care services that supports Canadians. We’re excited to offer this groundbreaking and innovative test as a means to support patients and oncologists greater clarity in making precise clinical decisions regarding cancer treatment(s).”
SignateraTM testing involves two phases with pre-supplied collection kits. The first phase is an initial test that analyzes both a tumour tissue and blood sample, and the second phase involves subsequent blood tests on an as-needed basis. It is a safe, non-invasive way to monitor ctDNA levels to help physicians understand treatment efficacy and detect relapse without the inconvenience of repeated tissue biopsies and/or imaging.
To learn more about the test, visit LifeLabs at https://www.lifelabsgenetics.com/product/signatera/.
If you have any questions or need assistance, you can contact 1-844-363-4357 (press 0), or Ask.Genetics@LifeLabs.com.
About LifeLabs
LifeLabs is Canada’s leading provider of laboratory diagnostic information and digital health connectivity systems, enabling patients and healthcare practitioners to diagnose, treat, monitor, and prevent disease. We support 20 million patient visits annually and conduct over 100 million laboratory tests through leading edge technologies and our 6,500 talented and dedicated employees. We are a committed innovator in supporting Canadians to live healthier lives, operating Canada’s first commercial genetics lab, and the country’s largest online patient portal, with more than 5 million Canadians receiving their results online. LifeLabs has been named one of Canada’s Best Employers (2021 and 2022) and Best Employers for Diversity (2022) by Forbes and recognized for having an award-winning Mental Health Program from Benefits Canada. LifeLabs is 100% Canadian owned by OMERS Infrastructure, the infrastructure investment manager of one of Canada’s largest defined benefit pension plans. Learn more at lifelabs.com.
About Natera
Natera™ is a global leader in cell-free DNA testing, dedicated to oncology, women’s health, and organ health. Our aim is to make personalized genetic testing and diagnostics part of the standard of care to protect health and enable earlier and more targeted interventions that help lead to longer, healthier lives. Natera’s tests are validated by more than 100 peer-reviewed publications that demonstrate high accuracy. Natera operates ISO 13485-certified and CAP-accredited laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) in Austin, Texas and San Carlos, California. For more information, visit www.natera.com.
*LifeLabs is in partnership with Natera to distribute and commercialize the Signatera molecular residual disease test in Canada. Natera conducts all sample testing and analysis.
Sources:
1) Reinert T, Henriksen TV, Christensen E, et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stage I to III Colorectal Cancer. JAMA Oncology. 2019;5(8):1124-1131.
2) Coombes C, Page K, Salari R, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clinical Cancer Research. 2019;25(14):4255-4263.
3) Abbosh C, Birkbak N, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017,545:446–451
4) Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients with Urothelial Bladder Carcinoma. 2019; 37(18):1547-1557.





