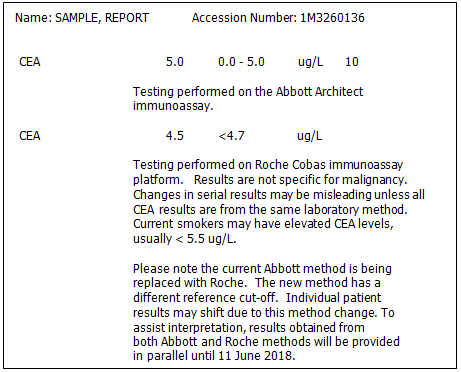
On December 11th, 2017 LifeLabs will introduce a new methodology for carcinoembrionic antigen (CEA) testing, based on the Roche Cobas system.
Please note that patients whose samples are sent to hospital laboratories with the Cancer Care Ontario CEA requisition form are not affected by this change.
The change will only affect patients whose CEA levels are currently monitored at LifeLabs.
Due to the methodology change there will be a new reference cut-off of “<4.7 µg/L” which will be noted on reports. Also, individual patient results may shift significantly. To assist result interpretation, LifeLabs will be testing all patient samples in parallel on the current Abbott Architect methodology and the new Roche Cobas methodology until June 11th, 2018. We strongly recommend that health care providers have their patients tested for CEA before this date (June 11th 2018) in order to obtain results from parallel testing, and thus have baseline results for continued monitoring of CEA levels.
An example of the patient report below indicates the information that will be provided on lab reports during the parallel testing period.
Please contact the LifeLabs Customer Care Centre 1-877-849-3637 for all enquiries. We welcome your feedback!
Patrick St. Louis PhD FCACB Chemistry Discipline Head