1.https://alzheimer.ca/sites/default/files/documents/Landmark-Study-Report-1-Path_Alzheimer-Society-Canada_0.pdf (accessed on Mar 20, 2025).
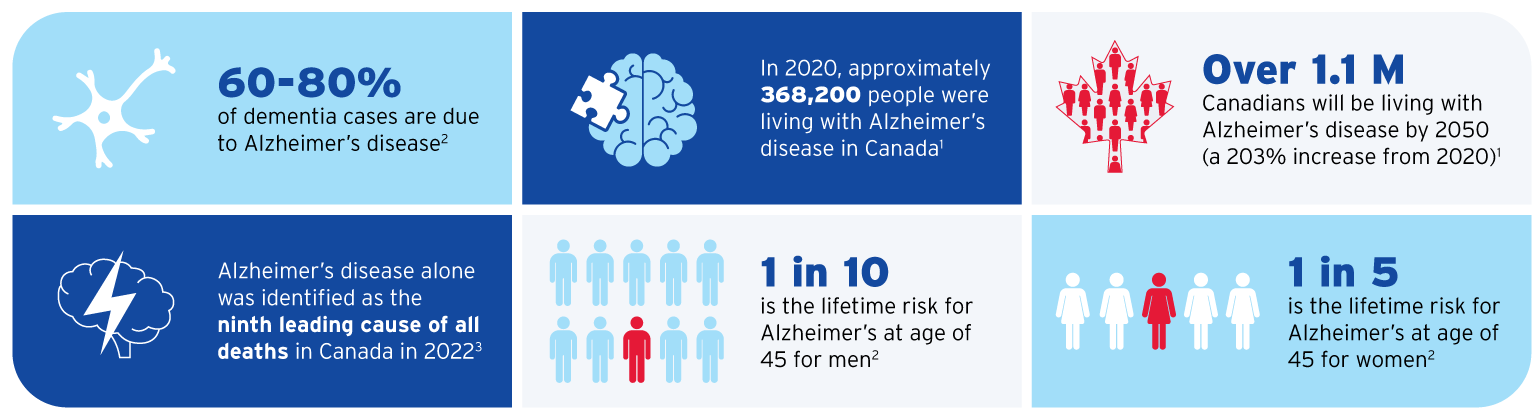
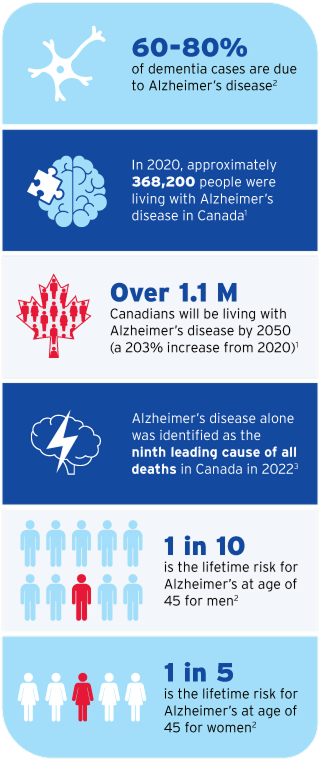
2. Alzheimer’s Association 2024 Alzheimer’s Disease Facts and Figures (accessed on Mar 20, 2025).
3. https://www.statcan.gc.ca/o1/en/plus/5374-alzheimers-awareness-month (accessed on Mar 20, 2025).
4. Thijssen EH, Joie RL, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739-752. doi:10.1016/s1474-4422(21)00214-3
5. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. doi:10.1001/jama.2020.12134
6. Ashton NJ, Brum WS, Molfetta GD, et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 2024;81(3):255-263. doi:10.1001/jamaneurol.2023.5319
7. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398-1405. doi:10.1038/s41591-022-01822-2
8. Therriault J, Ashton NJ, Pola I, et al. Comparison of two plasma p-tau217 assays to detect and monitor Alzheimer’s pathology. eBioMedicine. 2024;102:105046. doi:10.1016/j.ebiom.2024.105046
9. Nishimura M, Satoh M, Nishimura S, et al. Human apolipoprotein E resequencing by proteomic analysis and its application to serotyping. PLoS ONE. 2014;9(1):e85356. doi:10.1371/journal.pone.0085356
10. Kirmess KM, Meyer MR, Holubasch MS, et al. The PrecivityAD™ test: accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta. 2021;519:267-275. doi:10.1016/j.cca.2021.05.011
11. Nakamura A, Kaneko N, Villemagne V, et al. High performance plasma amyloid-B biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249-254. doi:10.1038/nature25456
12. Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to postmortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267-278. doi:10.1007/s00401-020-02195-x
13. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353-1364. doi:10.1002/alz.12301
14. Meyer PF, Ashton NJ, Karikari TK, et al. Plasma p-tau231, p-tau181, PET biomarkers, and cognitive change in older adults. Ann Neurol. 2022;91(4):548-560. doi:10.1002/ana.26308
15. Weber DM, Stroh MA, Taylor SW, et al. Development and clinical validation of blood-based multibiomarker models for the evaluation of brain amyloid pathology. medRxiv 2025;02.27. doi:10.1101/2025.02.27.25322892
16. Schindler SE, Galasko D, Pereira AC, et al. Acceptable performance of blood biomarker tests of amyloid pathology — Recommendations from the Global CEO Initiative on Alzheimer’s disease. Nat Rev Neurol. 2024;20(7):426-439. doi:10.1038/s41582-024-00977-5




 Return to collection guide
Return to collection guide